Science
TetraKit: The Universal Labeling Platform
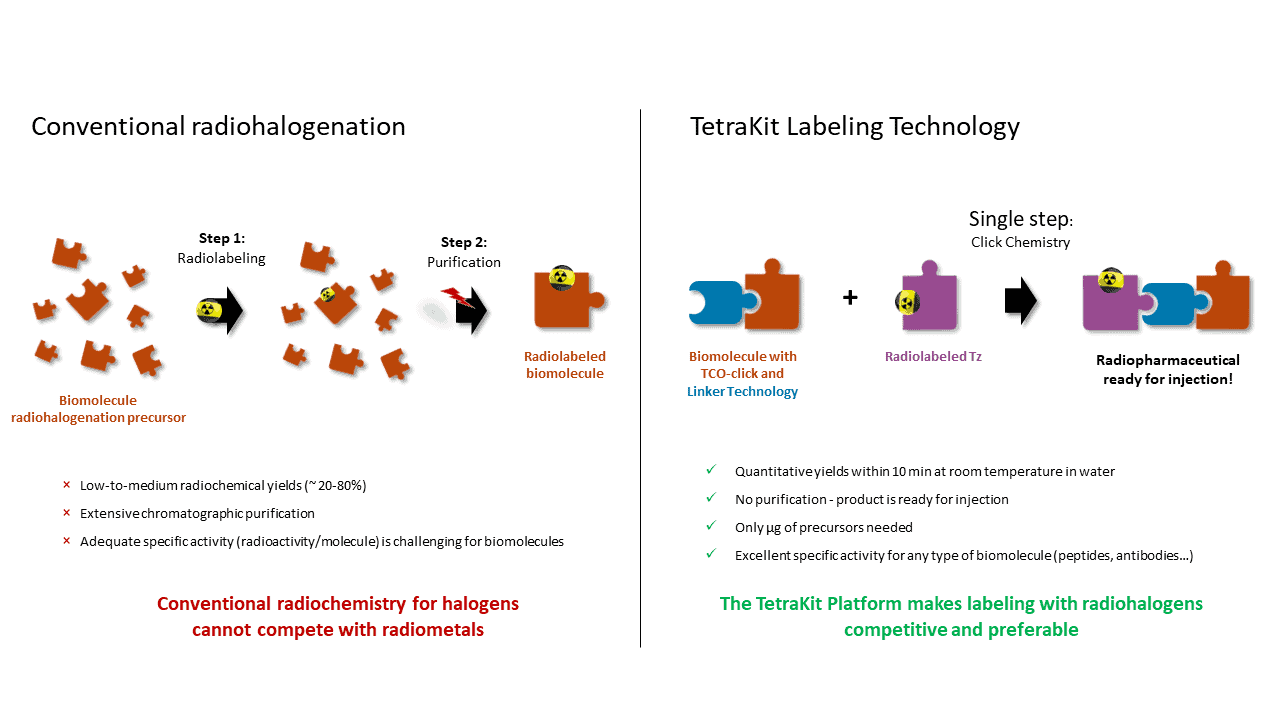
The TetraKit platform revolutionizes radiolabeling with its proprietary click chemistry, facilitating rapid and mild labeling conditions without requiring purification of the final product. This versatile approach is applicable across various molecules such as small molecules, peptides, antibodies, or nanoparticles, making it ideal for theranostic applications. Its kit-labeling strategy ensures easy implementation in radiopharmacies, enabling efficient production of radiopharmaceuticals. TetraKit's click chemistry offers quantitative labeling within minutes in water, eliminating the need for purification. This modular approach allows for plug-and-play radiolabeling of targeting ligands with radiohalogens, rivaling the convenience of current radiometal labeling methods. Its rapidity and adaptability extend to diverse targeting vector classes like peptides, proteins, or oligonucleotides, making it suitable for a wide range of radiopharmaceutical development. Furthermore, TetraKit's linker technology guides the biodistribution of radiolabeled drugs towards renal excretion, enhancing their therapeutic efficacy. Its competitive labeling capabilities with radiohalogens position it as a promising option for theranostic radionuclides, particularly where conventional radiochemistry falls short. This innovative platform is poised to advance radiopharmaceutical research and production, offering a streamlined and effective solution for medical imaging and therapy.
Theranostics
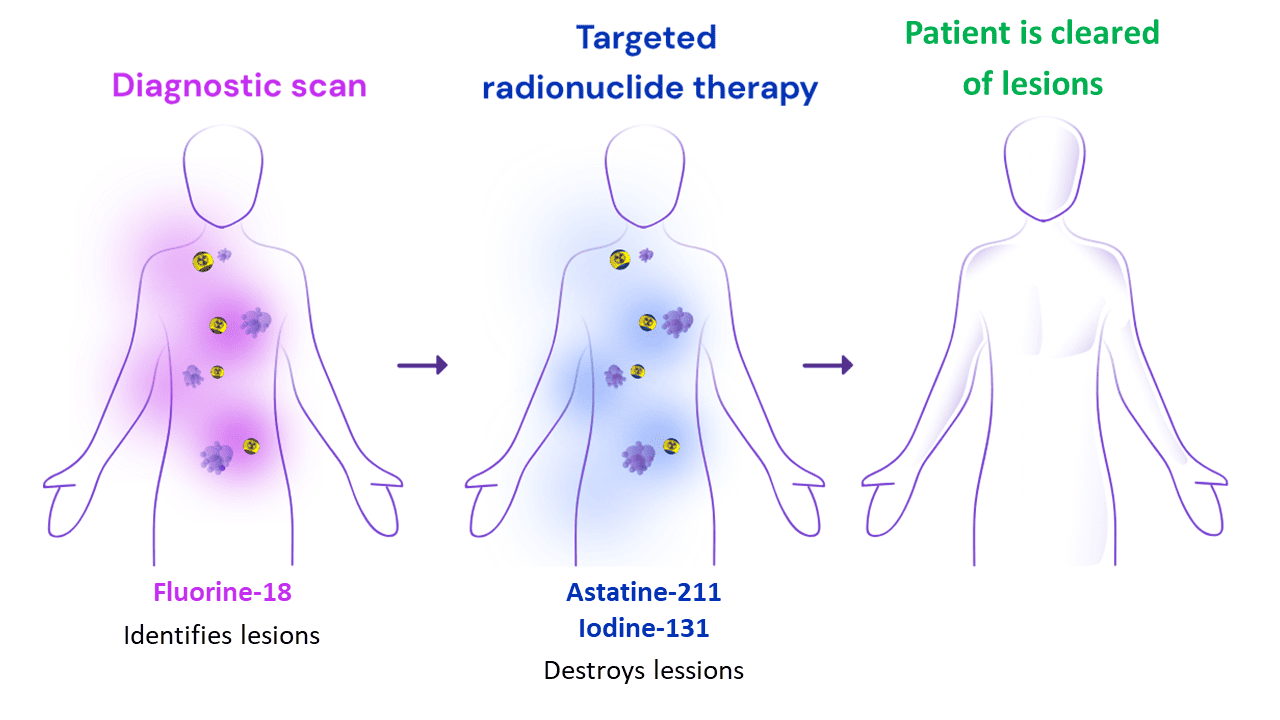
Theranostics is a new concept within clinical nuclear medicine, which combines radionuclide therapy with diagnostic nuclear imaging. Theranostics relies on complementary pairs of radiopharmaceuticals - compounds where a radionuclide is attached to a cancer targeting vector. Radiopharmaceuticals specifically target tumors when injected into a patient. In theranostics, the same targeting vector is labeled with radionuclides that have different properties. Diagnostic radionuclides, such fluorine-18, make it possible to identify tumor lesions by means of positron emission tomography (PET) imaging. Putting a therapeutic radionuclide, such as astatine-211, on the same vector allows to destroy the same tumor lesions by means of targeted radionuclide therapy (TAT). Theranostics is ideal for treating metastatic cancer, which is the main challenge in current cancer management and often the reason for cancer reoccurrence.
Positron emission tomography (PET) for diagnostic imaging
Positron emission tomography (PET) is the most advanced form of diagnostic nuclear imaging, increasingly dominant in the clinic since 1995. It employs radiolabeled molecules known as radiotracers, which are manufactured by chemically combining a cancer-targeting vector with a positron emitting radioisotope. After PET radiotracers are administered to the patient by intravenous injection, they recognize and bind to tumor cells and accumulate in tumors and metastases. The patient is then scanned with a PET scanner producing a three-dimensional, quantitative image. PET can detect tumor lesions long before CT, MRI or SPECT, which improves diagnostic precision and treatment outcomes.
Targeted alpha therapy
Targeted alpha therapy (TAT) relies on antibodies, peptides and other targeting vectors conjugated to alpha-emitting radionuclides. Like PET radiotracers, TAT agents target tumor lesions by recognizing specific receptors on cancer cells and binding to them.
Thanks to the short travel distance of alpha-particles (a few micrometers), TAT agents deliver a highly focused radiation dose to cancer cells and spare surrounding healthy tissue from radiation damage. Alpha-particles create double-strand DNA breaks, which lead to cell death. Therefore, alpha-emitters are more effective at killing cancer cells than beta-emitters which are currently applied in the clinic.